在抗体药物开发过程中细胞株开发是CMC的起点和基础,可以说CMC的大部分工作都是直接或间接与细胞株有关。稳定细胞株适用于各种研究应用, 如重组蛋白和重组抗体生产、检测分析、基因编辑、功能性研究等等。
百英可以提供直接用于CMC大规模生产的稳定细胞株开发服务,我们的CHO K1细胞株来源清晰,表达量高,来源ECACC,可以全球亚授权。

在抗体药物开发过程中细胞株开发是CMC的起点和基础,可以说CMC的大部分工作都是直接或间接与细胞株有关。稳定细胞株适用于各种研究应用, 如重组蛋白和重组抗体生产、检测分析、基因编辑、功能性研究等等。
百英可以提供直接用于CMC大规模生产的稳定细胞株开发服务,我们的CHO K1细胞株来源清晰,表达量高,来源ECACC,可以全球亚授权。

单抗保证大于4g/L(摇瓶)

10年以上细胞株开发经验,上百个细胞株项目开发

以公司为单位授权,一次授权,永久使用
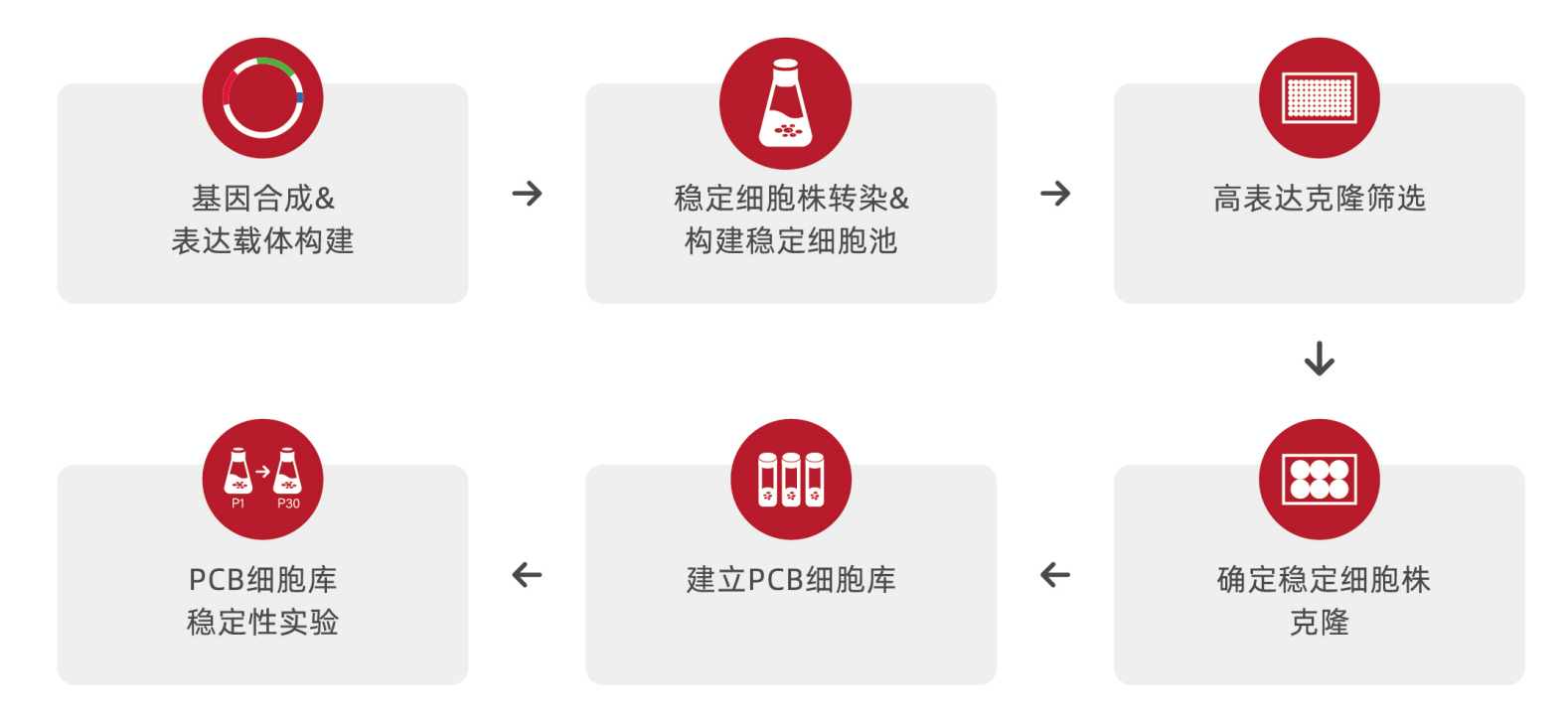
| 步骤 | 服务内容 | 周期 | 交付 |
|---|---|---|---|
| 选项1: CHO K1细胞株授权及项目开发 | |||
基因合成、亚克隆及表达纯化 | ▸密码子优化和基因合成 ▸亚克隆到表达质粒 ▸质粒验证、大量抽提及表达纯化 | 2 周 | ▸COA报告 |
| 细胞池构建 | ▸稳定转染 ▸细胞池筛选 | 6-9 周 | ▸50mL 上清液(细胞池) ▸详细的实验报告 |
| 单克隆筛选 | ▸亚克隆 ▸单克隆筛选 | 6-9 周 | |
| Primary Cell Bank 收集 | ▸Primary Cell Bank (PCB) 准备 ▸PCB 稳定性鉴定 | 12 周 | ▸Primary Cell Bank QC检测 ▸COA报告 ▸详细的实验报告 |
| CHO-K1 细胞亚授权 | ▸CHO-K1 细胞株报告 ▸CHO-K1 细胞亚授权协议 | 3-5 天 | ▸2管CHO-K1细胞株 ▸CHO-K1细胞株文件和检测报告 ▸CHO-K1 细胞亚授权协议 |
选项2: CHO-K1 细胞单独亚授权 | |||
| CHO-K1 细胞亚授权 | ▸CHO-K1 细胞株报告 ▸CHO-K1 细胞亚授权协议 | 3-5 天 | ▸2管CHO-K1细胞株 ▸CHO-K1细胞株文件和检测报告 ▸CHO-K1 细胞亚授权协议 |
|  |
| |
Monoclonality Proving
| |



上海市浦东新区周浦镇沈梅路99弄1号楼